💥New Chapter 11 Bankruptcy Filing - Eiger Biopharmaceuticals ($EIGR)💥
Commercial-stage biopharma company files to sell its therapies.
On April 1, 2024, CA-based Eiger BioPharmaceuticals Inc. ($EIGR)(“Eiger”) and four affiliates (collectively, the “debtors”) filed chapter 11 bankruptcy cases in the Northern District of Texas (Judge Jernigan).*
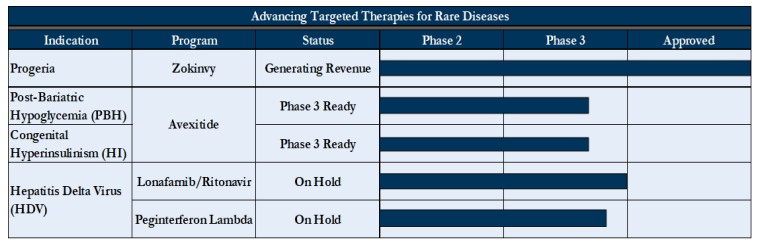
As the name suggests, Eiger BioPharmaceuticals ($EIGR) is a (commercial-stage) biopharma company focused on developing therapies for rare diseases. According to the FDA, a disease classifies as “rare” if it affects less than 200k people in the US. As of the filing, Eiger has one FDA-approved product and several others in clinical trial stages. The one FDA-approved product, Zokinvy, treats Hutchinson-Gilford Progeria Syndrome. Another, Avexitide, is in Phase 3 ready clinical trials, and is designed to treat post-bariatric hypoglycemia and other forms of hyperinsulinemic hypoglycemia arising after gastrointestinal surgeries. The debtors outline each of their products and phases in the following table:
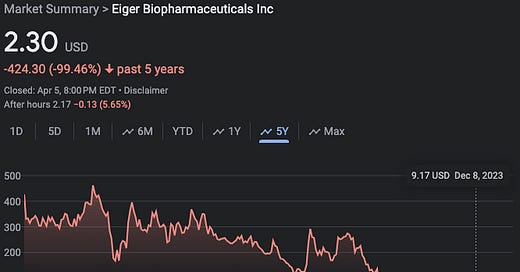
Founded in ‘08, Eiger went public in ‘16 after it completed a merger with Celladon Corporation. Life in the public markets has been … uh … quite a wild ride: